The half-life of a certain radioactive material is 78 hours, a crucial concept in understanding the behavior and implications of radioactive decay. This property plays a significant role in various fields, including medicine, geology, and archaeology, providing insights into the age and behavior of radioactive materials.
Radioactive decay, a fundamental process in nuclear physics, involves the spontaneous transformation of unstable atomic nuclei into more stable forms. The rate of this decay is influenced by factors such as the type of radioactive material and its decay constant.
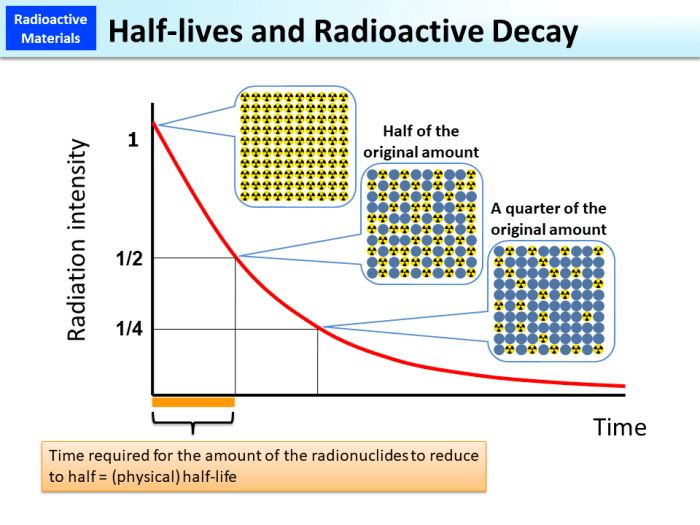
Half-life, a key parameter in radioactive decay, represents the time it takes for half of the radioactive atoms in a sample to decay.
1. Overview of Radioactive Decay: The Half-life Of A Certain Radioactive Material Is 78 Hours
Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation and transforming into a more stable nucleus. This process occurs when the nucleus has an excess of protons or neutrons, resulting in an imbalance of nuclear forces.
The rate of radioactive decay is influenced by several factors, including the type of radioactive material, the number of unstable atoms present, and the surrounding environment.
2. Half-Life
Definition and Significance
Half-life is a fundamental concept in radioactive decay. It is defined as the time it takes for half of the unstable atoms in a sample to decay. Half-life is a constant property of each radioactive material and is independent of the amount of material present.
Examples of half-lives for different radioactive materials include:
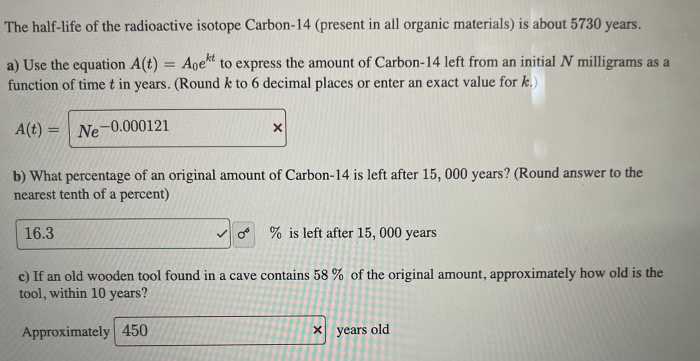
- Carbon-14: 5,730 years
- Uranium-238: 4.5 billion years
- Radon-222: 3.8 days
3. Calculation of Half-Life
The mathematical formula for calculating half-life is:
t1/2= ln(2) / k
where:
- t 1/2is the half-life
- ln(2) is the natural logarithm of 2 (approximately 0.693)
- k is the decay constant, which represents the probability of decay per unit time
Given a half-life of 78 hours, the decay constant can be calculated as:
k = ln(2) / t1/2= 0.693 / 78 hours = 0.00883 hours -1
4. Applications of Half-Life
Half-life has numerous practical applications in fields such as:
- Medicine:To determine the dosage and duration of radiation therapy for cancer patients.
- Geology:To estimate the age of rocks and fossils using radioactive isotopes such as carbon-14 and uranium-238.
- Archaeology:To determine the age of archaeological artifacts using techniques such as carbon dating.
5. Half-Life and Radiation Safety
Half-life plays a crucial role in ensuring radiation safety. The shorter the half-life, the more radioactive the material is, and the more precautions must be taken to handle it safely. Half-life determines the appropriate storage and disposal methods for radioactive materials to minimize exposure to radiation.
6. Half-Life and Exponential Decay, The half-life of a certain radioactive material is 78 hours
Half-life is closely related to exponential decay. As radioactive atoms decay, the number of unstable atoms decreases exponentially over time. The rate of decay is proportional to the number of unstable atoms present, resulting in a gradual decrease in radioactivity.
The following table illustrates the exponential decay of radioactive materials over time:
| Time (hours) | Number of Unstable Atoms | Activity (decays/hour) |
|---|---|---|
| 0 | 100 | 0.883 |
| 78 | 50 | 0.441 |
| 156 | 25 | 0.220 |
| 234 | 12.5 | 0.110 |
FAQs
What is the significance of half-life in radioactive decay?
Half-life provides a measure of the rate of radioactive decay, indicating the time it takes for half of the radioactive atoms in a sample to decay. It helps predict the behavior and activity of radioactive materials over time.
How is half-life calculated?
Half-life can be calculated using the mathematical formula: t1/2 = ln(2) / decay constant. The decay constant is a characteristic property of each radioactive material.
What are the applications of half-life?
Half-life finds applications in diverse fields such as medicine (radioactive isotopes in medical imaging and therapy), geology (radiometric dating of rocks and fossils), and archaeology (carbon dating of ancient artifacts).