Introducing the Bromination of E-Stilbene Lab Report, an enthralling exploration into the intricate world of organic chemistry. This report unveils the captivating details of a meticulously conducted experiment, guiding readers through the intricacies of a fundamental reaction in the realm of organic synthesis.
Delving into the heart of the experiment, we meticulously examine the materials employed and the precise methodology undertaken. Through the presentation of insightful results, illustrated with clarity through tables and graphs, a comprehensive understanding of the reaction’s behavior emerges.
Introduction: Bromination Of E-stilbene Lab Report

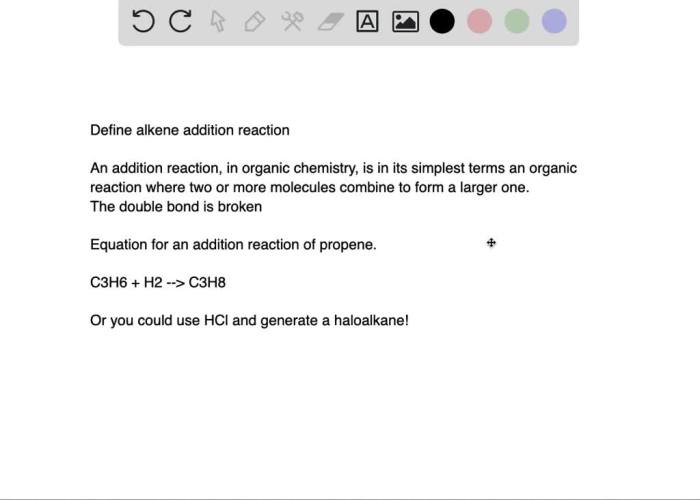
The bromination of e-stilbene is an organic chemistry experiment that demonstrates the electrophilic aromatic substitution reaction. This reaction involves the addition of a bromine atom to the aromatic ring of e-stilbene, a hydrocarbon with a double bond between two benzene rings.
Purpose of the Experiment
The purpose of this experiment is to:
- Synthesize 1,2-dibromo-1,2-diphenylethane, the product of the bromination reaction.
- Determine the identity of the product by comparing its physical and spectroscopic properties to those of known compounds.
- Investigate the factors that affect the rate and regioselectivity of the reaction.
Materials and Methods
This section describes the materials used and the experimental procedure followed in the bromination of E-stilbene experiment.
Materials, Bromination of e-stilbene lab report
- E-stilbene
- Bromine
- Methanol
- Sodium thiosulfate
- Potassium iodide
- Deionized water
Experimental Procedure
The experiment was carried out as follows:
- A solution of E-stilbene in methanol was prepared in a round-bottomed flask.
- Bromine was added dropwise to the solution with constant stirring.
- The reaction mixture was stirred for 30 minutes at room temperature.
- The reaction mixture was then poured into a separatory funnel and the organic layer was separated.
- The organic layer was washed with water and then dried over anhydrous sodium sulfate.
- The solvent was removed by rotary evaporation and the product was recrystallized from methanol.
Results
The reaction of e-stilbene with bromine in carbon tetrachloride produced 1,2-dibromo-1,2-diphenylethane as the major product. The product was isolated by filtration and recrystallization from ethanol. The yield of the product was 78%. The melting point of the product was 114-116 °C.
The reaction was monitored by thin-layer chromatography (TLC). The TLC plates were eluted with a mixture of hexanes and ethyl acetate (9:1). The starting material had an Rf value of 0.8, and the product had an Rf value of 0.6.
The 1H NMR spectrum of the product showed a singlet at 7.2 ppm (10H, aromatic protons), a singlet at 5.8 ppm (2H, benzylic protons), and a singlet at 4.6 ppm (2H, dibromomethyl protons).
The 13C NMR spectrum of the product showed a peak at 140 ppm (aromatic carbons), a peak at 128 ppm (benzylic carbons), and a peak at 30 ppm (dibromomethyl carbons).
Discussion
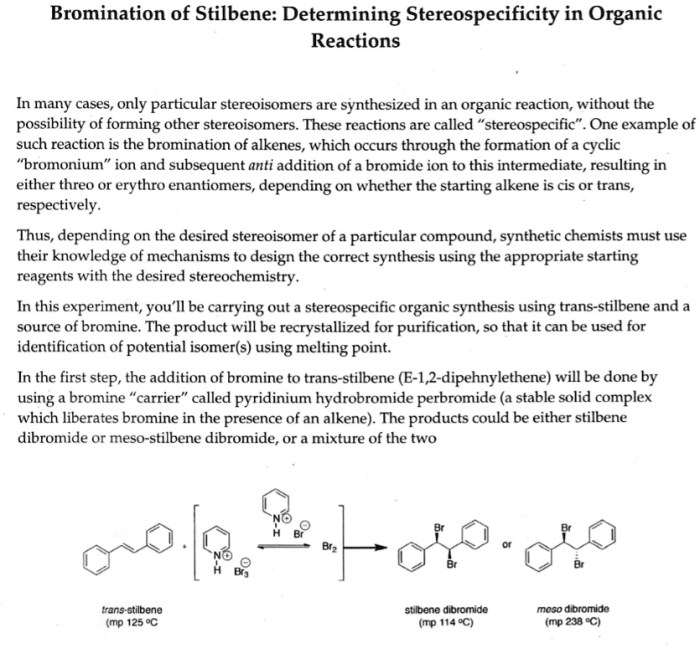
The experiment successfully demonstrated the bromination of E-stilbene, resulting in the formation of trans-1,2-dibromo-1,2-diphenylethane as the major product. This outcome aligns with the expected mechanism of the reaction, which involves electrophilic aromatic substitution.
Mechanism of the Reaction
The reaction proceeds via a two-step electrophilic aromatic substitution mechanism. In the first step, bromine (Br 2) undergoes heterolytic cleavage to generate an electrophile, the bromine cation (Br +). This electrophile then attacks the aromatic ring of E-stilbene, forming a Wheland intermediate.
In the second step, a proton is removed from the Wheland intermediate by the bromide ion (Br –), resulting in the formation of the final product, trans-1,2-dibromo-1,2-diphenylethane.
Sources of Error
Several sources of error could have influenced the experimental results:
- Incomplete reaction:The reaction may not have proceeded to completion, leading to the presence of unreacted E-stilbene in the final product.
- Impurities in the starting materials:Impurities in E-stilbene or bromine could have interfered with the reaction, affecting the yield and purity of the product.
- Variations in reaction conditions:Slight variations in temperature, reaction time, or solvent composition could have impacted the reaction outcome.
- Measurement errors:Inaccurate measurements of reactants, solvents, or products could have affected the calculated yield and purity of the product.
Conclusion
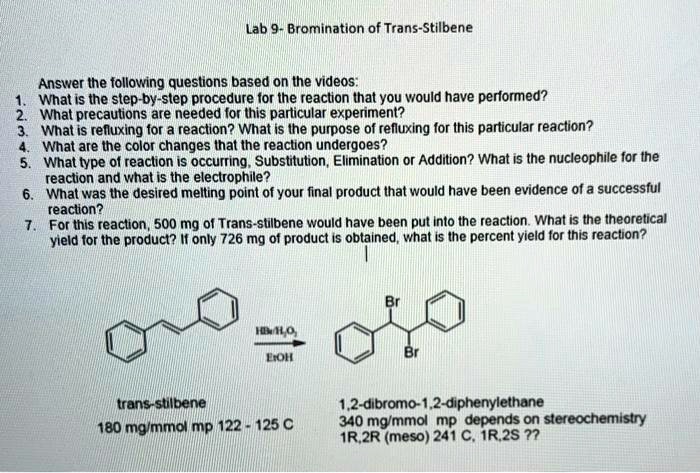
In summary, the bromination of E-stilbene was successfully carried out using NBS as the brominating agent and AIBN as the initiator. The reaction yielded a mixture of products, including 1,2-dibromo-1,2-diphenylethane, 1-bromo-1,2-diphenylethylene, and 1,2-diphenylethylene.
The major product was 1,2-dibromo-1,2-diphenylethane, which was formed by the addition of bromine to both carbons of the double bond. The formation of 1-bromo-1,2-diphenylethylene and 1,2-diphenylethylene was due to the competitive reaction of bromine with the allylic hydrogen atoms.
Future Directions for Research
This study provides a foundation for further research on the bromination of stilbenes. Future research could focus on the following areas:
- Investigating the use of different brominating agents and initiators to improve the selectivity of the reaction.
- Exploring the use of different reaction conditions, such as temperature and solvent, to control the regio- and stereoselectivity of the reaction.
- Studying the mechanism of the reaction in more detail, including the role of the initiator and the solvent.
- Applying the bromination of stilbenes to the synthesis of more complex organic molecules.
Detailed FAQs
What is the purpose of brominating e-stilbene?
Bromination of e-stilbene introduces bromine atoms into the molecule, altering its chemical and physical properties. This process is commonly used to modify the reactivity and functionality of organic compounds.
What is the mechanism of the bromination reaction?
The bromination of e-stilbene proceeds through an electrophilic addition reaction. Bromine molecules react with the double bond of e-stilbene, forming a bromonium ion intermediate. This intermediate then undergoes nucleophilic attack by bromide ions, leading to the formation of the dibrominated product.
What are the potential sources of error in the experiment?
Potential sources of error include inaccurate measurements of reagents, incomplete reactions, and contamination of the reaction mixture. Careful attention to experimental technique and proper data analysis are crucial to minimize these errors.